Section 2H: Complete your Formula of a Hydrate lab and then watch and take notes on this video: How to Calculate Limiting Reactant
Section 5: Complete the stoichiometry worksheet we've been working on (questions 1-5 all parts). Also, try these:
1) 8 Fe + S8 ---> 8 FeS
a) What mass of iron is needed to react with 16.0 grams of sulfur?
b) How many grams of FeS are produced?
2) Cu + 2 AgNO3 ---> Cu(NO3)2 + 2 Ag
a) How many moles of Cu are needed to react with 3.50 moles of AgNO3?
b) If 89.5 grams of Ag were produced, how many grams of Cu reacted?
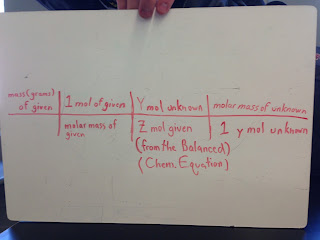
This great template, courtesy of Jack Kitson, Jeremy Ayala and Ryan Coad should help: