Sections 1, 2 & 6:
1) Quia assignment will be up until tomorrow morning.
2) Make sure you have your balancing equations worksheet (from the end of class today) and your lab ready for the Monday that we return.
3) Extra credit can be found under homework docs, it is due on Monday in class...good luck!
Section 7:
1) Quia assignment will be up until tomorrow morning.
2) Make sure you have yesterday's homework and your lab ready for the Monday that we return.
3) Extra credit can be found under homework docs, it is due on Monday in class...good luck!
All: There are a few questions in the midterm review packet (about types of chemical reactions) that we will cover when we get back from break. Skip those questions for now!
Happy Holidays!!
Website Image

Wednesday, December 18
Sections 1, 2 & 6: Complete the worksheet we started in class and the 20 balancing chemical equations problems under 'homework documents'
Section 3:
1) Don't forget to pick up the answer key and extra credit assignment on my office door tomorrow
2) You will be emailed the answer key to the balancing homework
3) Read pages 276 - 284 in your textbook and answer questions 1-4 on page 284
4) Quia HW will be open from today until Friday morning!
4) Quia HW will be open from today until Friday morning!
Section 7:
1) Get your data from a classmate
2) You will be emailed the answer key to last night's homework
3) Read pages 276 - 284 in your textbook and answer questions 1-4 on page 284
All: Here's a video on balancing equations, if you need some extra help:
Balancing Chemical Equations
All: Here's a video on balancing equations, if you need some extra help:
Balancing Chemical Equations
Posted by
Ms. Tanzman
Tuesday, December 17
Sections 1, 2 & 6: Watch and take notes on the following video:
Chemical Equation Writing
Sections 3 & 7: Complete the 20 balancing chemical equations problems under 'homework documents'
Chemical Equation Writing
Sections 3 & 7: Complete the 20 balancing chemical equations problems under 'homework documents'
Posted by
Ms. Tanzman
Monday, December 16
Sections 1 & 2: Make sure to get data/observations for every station from someone in class.
Sections 3 & 7: Watch and take notes on the following video:
Chemical Equation Writing
Sections 3 & 7: Watch and take notes on the following video:
Chemical Equation Writing
Posted by
Ms. Tanzman
Friday, December 13
UPDATE: I have posted data for stations A, B, C, D & E under the homework docs section.
Sections 3 & 6: Make sure to email me the photo of your observations from your station! I will post them as soon as I get them!
Also, don't forget to see the show this weekend...it looks awesome..
One Flew Over the Cuckoo's Nest
Sections 3 & 6: Make sure to email me the photo of your observations from your station! I will post them as soon as I get them!
Also, don't forget to see the show this weekend...it looks awesome..
One Flew Over the Cuckoo's Nest
Posted by
Ms. Tanzman
Thursday, November 12
All Sections: You can re-do your quia quiz tonight - if you're having issues, please email me BEFORE 9pm.
Also, read and take notes on pages 201-206 in your textbook and answer questions 1-6 on page 207 (you will need more info than just the info on pages 201-206, refer back to your other notes/earlier pages in the book).
Also, read and take notes on pages 201-206 in your textbook and answer questions 1-6 on page 207 (you will need more info than just the info on pages 201-206, refer back to your other notes/earlier pages in the book).
Posted by
Ms. Tanzman
Friday, December 6
All Sections: Here's everything that needs to be done before class on Thursday:
1) Watch and take notes on this video:
Covalent And Ionic Bonding
Also, this video will help you understand VSEPR theory: VSEPR theory intro
2) Complete all of the worksheets found under 'Homework Documents'. Many of them you already received in hard copy in class, but this is a way to make sure you've completed everything.
3) Compete the Quia assignment, which can be found on your class page. It is titled 'Ionic and Covalent Bonding'. You may take it up to 3 times. You will have 45 minutes for each attempt. This must be done by Wednesday at midnight.
Make sure to bring your worksheets with you to class so that you can work with your tablemates!
See you on Thursday!
1) Watch and take notes on this video:
Covalent And Ionic Bonding
Also, this video will help you understand VSEPR theory: VSEPR theory intro
2) Complete all of the worksheets found under 'Homework Documents'. Many of them you already received in hard copy in class, but this is a way to make sure you've completed everything.
3) Compete the Quia assignment, which can be found on your class page. It is titled 'Ionic and Covalent Bonding'. You may take it up to 3 times. You will have 45 minutes for each attempt. This must be done by Wednesday at midnight.
Make sure to bring your worksheets with you to class so that you can work with your tablemates!
See you on Thursday!
Posted by
Ms. Tanzman
Thursday, December 5
Sections 3 & 6: Complete the back of the covalent naming worksheet (Lewis structures). Also, use pages 197 - 203 in your textbook to complete the worksheet handed out at the end of class.
All: Here's another way of thinking about Lewis structures, if my method doesn't work for you:
https://www.youtube.com/watch?v=PtMifU1py5I
All: Here's another way of thinking about Lewis structures, if my method doesn't work for you:
https://www.youtube.com/watch?v=PtMifU1py5I
Posted by
Ms. Tanzman
Wednesday, December 4
Sections 1, 2, 6 & 7: Complete the Molecular Model Online Lab as we discussed in class. Other than the 3 websites given, you will also find pages 197-200 in your textbook quite helpful in naming the shapes.
Also, if you go to the 'Large Molecule' tab on the simulator, you will see there are 7 kits available, making it possible for you to do every compound on the worksheet. The images below should help you find the kits.
Finally, there is a practice worksheet for the compound naming quiz under homework docs.
Section 3: Complete the two naming worksheets we started in class today. Also, here's the link for creating your sketches for the Molecular Model Online Lab:
Build a Molecule
Also, if you go to the 'Large Molecule' tab on the simulator, you will see there are 7 kits available, making it possible for you to do every compound on the worksheet. The images below should help you find the kits.
Finally, there is a practice worksheet for the compound naming quiz under homework docs.
Section 3: Complete the two naming worksheets we started in class today. Also, here's the link for creating your sketches for the Molecular Model Online Lab:
Build a Molecule
Posted by
Ms. Tanzman
Tuesday, December 3
Sections 1, 2 & 6:
1) Complete the worksheet you got at the end of class today.
2) Read and take notes on pages 178-179 & 181-182 (stop @ Octet Rule) in your textbook.
3) Mess around with this SIM:
Build a Molecule
Once you've built something, you can look at it in '3D' or ball and stick model (see below for more help). If you see anything interesting, write it down in your notebook!
Section 7:
1) Complete the following problems in your notebook:
A) Name the following:
K2CO3
Be(OH)2
1) Complete the worksheet you got at the end of class today.
2) Read and take notes on pages 178-179 & 181-182 (stop @ Octet Rule) in your textbook.
3) Mess around with this SIM:
Build a Molecule
Once you've built something, you can look at it in '3D' or ball and stick model (see below for more help). If you see anything interesting, write it down in your notebook!
Section 7:
1) Complete the following problems in your notebook:
A) Name the following:
K2CO3
Be(OH)2
Cu2S
ZnI2
FePO4
A) Write the formulas for the following:
ammonium sulfate
lead (II) nitrite
iron (II) oxide
lead (IV) sulfite
magnesium iodide
2) Read and take notes on pages 178-179 & 181-182 (stop @ Octet Rule) in your textbook.
3) Mess around with this SIM:
Build a Molecule
Once you've built something, you can look at it in '3D' or ball and stick model (see above for more help). If you see anything interesting, write it down in your notebook!
2) Read and take notes on pages 178-179 & 181-182 (stop @ Octet Rule) in your textbook.
3) Mess around with this SIM:
Build a Molecule
Once you've built something, you can look at it in '3D' or ball and stick model (see above for more help). If you see anything interesting, write it down in your notebook!
Posted by
Ms. Tanzman
Monday, December 2
Sections 1, 3, 6 & 7: Quiz correction are due tomorrow. Also, finish your lab - ALL parts (including the back page!).
Section 2: Finish your lab - ALL parts (including the back page!).
Section 2: Finish your lab - ALL parts (including the back page!).
Welcome back! Winter is upon us!
Posted by
Ms. Tanzman
Happy Thanksgiving!
Enjoy your Thanksgiving!
You have no chemistry homework, but when you're DESPERATELY missing science, there's this:
True Facts: Chameleon
Or this:
Great Science Questions...Answered!
Or this:
Slo Mo Shaolin Warriors
OR THIS:
Will We Ever Run Out of Music?
You get the point...eat some turkey.. have fun.. think about the world around you... think about science...because....

You have no chemistry homework, but when you're DESPERATELY missing science, there's this:
True Facts: Chameleon
Or this:
Great Science Questions...Answered!
Or this:
Slo Mo Shaolin Warriors
OR THIS:
Will We Ever Run Out of Music?
You get the point...eat some turkey.. have fun.. think about the world around you... think about science...because....

Posted by
Ms. Tanzman
Monday, November 25
Section 1: Complete the following in the packet I handed out in class today:
Page 1 - All
Page 2- 2, 3, 4, 7
Page 3- 11, 13, 14, 15, 17, 18, 19, 22, 23
And - STUDY your TRENDS!
Section 2: Complete the following in the packet I handed out in class today:
Page 1 - 1-4
Page 2- 2, 3, 4
Also, draw the formula for the following ionic compounds in your notebook:
1) Potassium sulfide
2) Magnesium nitride
3) Barium Oxide
4) Lithium Chloride
And - STUDY your TRENDS!
Sections 3, 6 & 7: Study your trends. The videos for the bonus questions are below. You may also want to consult the packet that I gave out in class to help you prepare for any bonus questions. DO NOT LOSE THAT PACKET - WE WILL USE IT WHEN WE RETURN FROM BREAK.
All: Here are some helpful videos:
Writing Ionic Formulas: Introduction
Writing Formulas with Polyatomic Ions
**Quality Ch. 5 Outlines can be found at the bottom of the page!**
*I'll be in 4K3 at 7:40 tomorrow*
Page 1 - All
Page 2- 2, 3, 4, 7
Page 3- 11, 13, 14, 15, 17, 18, 19, 22, 23
And - STUDY your TRENDS!
Section 2: Complete the following in the packet I handed out in class today:
Page 1 - 1-4
Page 2- 2, 3, 4
Also, draw the formula for the following ionic compounds in your notebook:
1) Potassium sulfide
2) Magnesium nitride
3) Barium Oxide
4) Lithium Chloride
And - STUDY your TRENDS!
Sections 3, 6 & 7: Study your trends. The videos for the bonus questions are below. You may also want to consult the packet that I gave out in class to help you prepare for any bonus questions. DO NOT LOSE THAT PACKET - WE WILL USE IT WHEN WE RETURN FROM BREAK.
All: Here are some helpful videos:
Writing Ionic Formulas: Introduction
Writing Formulas with Polyatomic Ions
**Quality Ch. 5 Outlines can be found at the bottom of the page!**
*I'll be in 4K3 at 7:40 tomorrow*
Posted by
Ms. Tanzman
Thursday, November 21
Sections 3: Watch and take notes on the following video:
Trends and Lewis Structures
Also, complete all worksheets given out during class today
Section 6: Watch the videos below so that you can complete the worksheets I handed out last class:
Writing Ionic Formulas: Introduction
Writing Formulas with Polyatomic Ions
My sincere apologies to Taylor Ene, as these videos do not include my voice.
Sorry for the delay! Also, here's the image from class:
Trends and Lewis Structures
Also, complete all worksheets given out during class today
Section 6: Watch the videos below so that you can complete the worksheets I handed out last class:
Writing Ionic Formulas: Introduction
Writing Formulas with Polyatomic Ions
My sincere apologies to Taylor Ene, as these videos do not include my voice.
Sorry for the delay! Also, here's the image from class:
Posted by
Ms. Tanzman
Wednesday, November 20
Sections 1, 2, 6 & 7: Watch and take notes on the following video:
Trends and Lewis Structures
Section 3: Answer all of the following questions in your notebook:
1. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium.
2. Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum.
3. Rank the following elements by increasing electron affinity: sulfur, oxygen, neon, aluminum.
4. Explain why fluorine has a higher ionization energy than iodine.
5. What is the relationship between atomic radius and ionization energy?
6. Why does removing an electron from an atom (ionization energy) require an input of energy?
7. What trend in atomic radius occurs down a group on the periodic table? What causes this trend?
8. What trend in atomic radius occurs across a period on the periodic table? What causes this trend?
9. Indicate whether the following properties increase or decrease from left to right across the
periodic table.
a. atomic radius (excluding noble gases)
b. first ionization energy
c. electronegativity
d. electron affinity
10. Why do you think elements in the same family generally have similar properties?
Trends and Lewis Structures
Section 3: Answer all of the following questions in your notebook:
1. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium.
2. Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum.
3. Rank the following elements by increasing electron affinity: sulfur, oxygen, neon, aluminum.
4. Explain why fluorine has a higher ionization energy than iodine.
5. What is the relationship between atomic radius and ionization energy?
6. Why does removing an electron from an atom (ionization energy) require an input of energy?
7. What trend in atomic radius occurs down a group on the periodic table? What causes this trend?
8. What trend in atomic radius occurs across a period on the periodic table? What causes this trend?
9. Indicate whether the following properties increase or decrease from left to right across the
periodic table.
a. atomic radius (excluding noble gases)
b. first ionization energy
c. electronegativity
d. electron affinity
10. Why do you think elements in the same family generally have similar properties?
Posted by
Ms. Tanzman
Tuesday, November 19
All: Answer all of the following questions in your notebook:
1. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium.
2. Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum.
3. Rank the following elements by increasing electron affinity: sulfur, oxygen, neon, aluminum.
4. Explain why fluorine has a higher ionization energy than iodine.
5. What is the relationship between atomic radius and ionization energy?
6. Why does removing an electron from an atom (ionization energy) require an input of energy?
1. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium.
2. Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum.
3. Rank the following elements by increasing electron affinity: sulfur, oxygen, neon, aluminum.
4. Explain why fluorine has a higher ionization energy than iodine.
5. What is the relationship between atomic radius and ionization energy?
6. Why does removing an electron from an atom (ionization energy) require an input of energy?
7. What trend in atomic radius occurs down a group on the periodic table? What causes this trend?
8. What trend in atomic radius occurs across a period on the periodic table? What causes this trend?
9. Indicate whether the following properties increase or decrease from left to right across the
periodic table.
a. atomic radius (excluding noble gases)
b. first ionization energy
c. electronegativity
d. electron affinity
10. Why do you think elements in the same family generally have similar properties?
Posted by
Ms. Tanzman
Monday, November 18
All: Lab reports for each group are due tomorrow (outline can be found at the bottom of the page). I want one HARD copy (non-digital) from each group. Also, don't forget that the Science Research Project packet that I handed you in the beginning of the year is a great resource!
**We will have a QUEST next Tuesday (11/26) in all sections**
**We will have a QUEST next Tuesday (11/26) in all sections**
Posted by
Ms. Tanzman
Tuesday, November 12
All: Below is the summary of all work to be completed while I'm away:
1) Test corrections should be done on loose-leaf, stapled to the front of your test and left in the box in the HMO by Friday.
2) Lab reports for each group are due on Tuesday (outline can be found at the bottom of the page). Also, don't forget that the Science Research Project packet that I handed you in the beginning of the year is a great resource!!
3) Periodic Trends project is due on Monday. Each project must include an outline of pages 150-164 in your textbook, and a visual representation of periodic trends. Please see the bottom of the page for more info.
1) Test corrections should be done on loose-leaf, stapled to the front of your test and left in the box in the HMO by Friday.
2) Lab reports for each group are due on Tuesday (outline can be found at the bottom of the page). Also, don't forget that the Science Research Project packet that I handed you in the beginning of the year is a great resource!!
3) Periodic Trends project is due on Monday. Each project must include an outline of pages 150-164 in your textbook, and a visual representation of periodic trends. Please see the bottom of the page for more info.
Posted by
Ms. Tanzman
Wednesday, November 6
All: Below you will find the problems that were on the board during class today. The answer key can be found under Homework Documents. PLEASE NOTE that you will not be given the equations on the test, only the constants.
Other study tools can also be found at the bottom of the page.
E=hv c=λv
h = 6.6262 x 10-34
J∙s
c = 3.0 x 108 m/s
Posted by
Ms. Tanzman
Tuesday, November 5
Sections 1, 2 & 7: Complete all of the sections from the packet that I assigned at the end of class today (if you were not savvy enough to write them down - phone a friend!)
Section 3: Complete the work from the packet assigned in class today AND print and complete the packet at the bottom of the page (if you didn't pick up a hard copy in class).

Section 3: Complete the work from the packet assigned in class today AND print and complete the packet at the bottom of the page (if you didn't pick up a hard copy in class).
All: Test on Thursday!!
Posted by
Ms. Tanzman
Monday, November 4
Section 3: As we discussed in class, you are to finish the worksheet we started today in class, and re-do any of the work from Friday that you think you did incorrectly
Section 6: Complete all of the sections from the packet that I assigned at the end of class today (if you were not savvy enough to write them down - phone a friend!)
Section 6: Complete all of the sections from the packet that I assigned at the end of class today (if you were not savvy enough to write them down - phone a friend!)
Posted by
Ms. Tanzman
Friday, November 1
Section 6: Complete 1-6 on the back of the worksheet we started in class. In the packet, complete one page 1 #1-6, page 2 #1-4, 11, 12, 16, 17 and page 3 #1-10.
Posted by
Ms. Tanzman
Thursday, October 31
Sections 1, 2 & 7: Complete #1-6 on the Electron Configuration Practice Worksheet. Also, complete the following in the homework packet: 1 (all parts), 5 (e- configuration only), 6 (a&b)
Section 6: Finish your lab AND take notes on the following video:
Electron Configurations

Section 6: Finish your lab AND take notes on the following video:
Electron Configurations
Posted by
Ms. Tanzman
Wednesday, October 30
All Sections: Create an outline of all of your notes from the chapter thus far (use the powerpoint below as a reference guide). We started this unit on Friday, October 4th (if you want to use the blog posts to help you recall everything we've covered). Include all pertinent information from videos, textbook reading, classroom notes and other miscellaneous homework. Outlines MUST be emailed to me by MIDNIGHT tonight.
Posted by
Ms. Tanzman
Tuesday, October 29
Section 1: Finish your lab and read pages 104-110 in your textbook. Put the definitions of all bolded words into your notes. Also, answer section review problems 1-4 on page 110.
Sections 2 & 6: Read pages 104-110 in your textbook. Put the definitions of all bolded words into your notes. Also, answer section review problems 1-4 on page 110.
Sections 3 & 7: Use the reading and your notes from last night's homework to answer questions 16, 17, 18 & 20 on pages 124 and 125 of your textbook.
Sections 3 & 7: Use the reading and your notes from last night's homework to answer questions 16, 17, 18 & 20 on pages 124 and 125 of your textbook.
Posted by
Ms. Tanzman
Monday, October 28
Sections 1 & 2: Read pages 100-103 in your textbook and answer section review questions 4,5 & 6 (all parts). Also, don't forget to use the powerpoint to evaluate the quality of your notes.
Finally, here's the video we watched today in class, if you want to watch it again:
Hydrogen Emission Spectrum
Sections 3 & 7: Read pages 104-110 in your textbook. Put the definitions of all bolded words into your notes. Also, answer section review problems 1-4 on page 110.
Sections 3 & 7: Read pages 104-110 in your textbook. Put the definitions of all bolded words into your notes. Also, answer section review problems 1-4 on page 110.
Posted by
Ms. Tanzman
Wednesday, October 23
Sections 3 & 6: Read pages 100-103 in your textbook and answer section review questions 4,5 & 6 (all parts)

Happy Mole Day!!
Posted by
Ms. Tanzman
Tuesday, October 22
Sections 1,2 & 6: Look at the webpage below and take notes on the content into your notebook. Try your best to answer the 'exercise' questions at the bottom of the page. You do not need to go to the next lesson.
http://skyserver.sdss.org/dr1/en/proj/advanced/spectraltypes/energylevels.asp
Sections 3: Watch the following video and take notes. The other website below will help you flesh out the quality/quantity of your notes:
https://www.youtube.com/watch?v=QI50GBUJ48s
.
http://skyserver.sdss.org/dr1/en/proj/advanced/spectraltypes/energylevels.asp
Try your best to answer the 'exercise' questions at the bottom of the page. You do not need to go to the next lesson
Section 7: Read pages 100-103 in your textbook and answer section review questions 4,5 & 6 (all parts)
http://skyserver.sdss.org/dr1/en/proj/advanced/spectraltypes/energylevels.asp
Sections 3: Watch the following video and take notes. The other website below will help you flesh out the quality/quantity of your notes:
https://www.youtube.com/watch?v=QI50GBUJ48s
.
http://skyserver.sdss.org/dr1/en/proj/advanced/spectraltypes/energylevels.asp
Try your best to answer the 'exercise' questions at the bottom of the page. You do not need to go to the next lesson
Section 7: Read pages 100-103 in your textbook and answer section review questions 4,5 & 6 (all parts)
Posted by
Ms. Tanzman
Monday, October 21
Sections 1 & 2: Watch the two videos below. They are VERY challenging to comprehend. Make sure to watch them more than once.
Section 6: A new assignment has been added to your Quia Class Page. Complete it by next class. Also, watch the two videos below. They are VERY challenging to comprehend. Make sure to watch them more than once.
Section 7: A new assignment has been added to your Quia Class Page. Complete it by next class.
Also, look at the webpage below and take notes on the content into your notebook. Try your best to answer the 'exercise' questions at the bottom of the page. You do not need to go to the next lesson.
Posted by
Ms. Tanzman
Friday, October 18
Section 1: A new assignment has been added to your Quia Class Page. Complete it by class on Monday.
Posted by
Ms. Tanzman
Thursday, October 17
All Sections: Watch and take notes/answer the question in the following video:
http://www.educreations.com/lesson/view/c-wavelength-x-frequency/12102329/?ref=appemail
http://www.educreations.com/lesson/view/c-wavelength-x-frequency/12102329/?ref=appemail
Posted by
Ms. Tanzman
Tuesday, October 15
Sections 3 & 7: Watch the two videos below. They are VERY challenging to comprehend. Make sure to watch them more than once.
Origin of Quantum Mechanics
QM History
Origin of Quantum Mechanics
QM History
Posted by
Ms. Tanzman
Friday, October 11
Section 3:
- Complete the electromagnetic spectrum worksheet at the bottom of the page
- Watch the first video (ONLY up to 5:30) and take notes on it. Then watch the second video, you do not need to take notes. Finally, answer the three questions below in your notebook.
Photoelectric Effect Demo
1) What is the relationship between the frequency of light and the energy of the electrons emitted?
2) The energy hitting the electrons is light energy, but the electrons leaving the metal are not made of light. What type of energy have those electrons transferred the light into?
3) What do you think this has to do with the problems we've discussed with Rutherford model of the atom?
Section 6: Work on your background research!
Posted by
Ms. Tanzman
Thursday, October 10
Sections 1, 2, 6 & 7: Watch the first video (ONLY up to 5:30) and take notes on it. Then watch the second video, you do not need to take notes. Finally, answer the three questions below in your notebook.
The Photoelectric Effect Explained
Photoelectric Effect Demo
1) What is the relationship between the frequency of light and the energy of the electrons emitted?
2) The energy hitting the electrons is light energy, but the electrons leaving the metal are not made of light. What type of energy have those electrons transferred the light into?
3) What do you think this has to do with the problems we've discussed with Rutherford model of the atom?
The Photoelectric Effect Explained
Photoelectric Effect Demo
1) What is the relationship between the frequency of light and the energy of the electrons emitted?
2) The energy hitting the electrons is light energy, but the electrons leaving the metal are not made of light. What type of energy have those electrons transferred the light into?
3) What do you think this has to do with the problems we've discussed with Rutherford model of the atom?
Posted by
Ms. Tanzman
Wednesday, October 9
Sorry I can't be with you guys today. If your class was canceled, the worksheet (Electromagnetic spectrum) you need to complete can be found under homework docs. You may use your textbook, the powerpoint at the bottom of the page or the internet to do so. You will need to do research to find most of the answers.
Also, there may be a box set up in the HMO for you to hand in your project. Please double check if there's a place to drop it off, rather than just holding on to it. If there is a drop off spot and you wait until tomorrow to hand it in, it may be considered late.
Also, there may be a box set up in the HMO for you to hand in your project. Please double check if there's a place to drop it off, rather than just holding on to it. If there is a drop off spot and you wait until tomorrow to hand it in, it may be considered late.
Posted by
Ms. Tanzman
Tuesday, October 8
Sections 1, 2, 3 & 7: Watch and take notes on the video below. There is A LOT of information in a very short amount of time. I suggest you watch it through once and then watch it again to pause and take notes in your notebook.
Electromagnetic Spectrum
Section 6: Here's the SIM for completing the worksheet (which can be found under homework docs at the bottom of the page):
Build An Atom Simulation
**Remember you can print and fill out the worksheet or write your answers on loose leaf or a combo of the two, just make sure it can be collected!
Electromagnetic Spectrum
Section 6: Here's the SIM for completing the worksheet (which can be found under homework docs at the bottom of the page):
Build An Atom Simulation
**Remember you can print and fill out the worksheet or write your answers on loose leaf or a combo of the two, just make sure it can be collected!
Posted by
Ms. Tanzman
Monday, October 7
All Sections: Watch the video below and use it to fill in the first 3 sections of the History of the Atom worksheet under 'Homework Documents'. You may also use the textbook (pgs. 67-76) and the internet to complete the assignment.
Early Atomic History
Early Atomic History
Posted by
Ms. Tanzman
Friday, October 4
Sections 1, 2 & 7: Watch the following video and take notes:
The Atom: Part 1
Here's the SIM for finishing the worksheet:
Build An Atom Simulation
Make sure you have all the materials/a plan for working your SRP on Monday.
Sections 3:
Here's the SIM for completing the worksheet:
Build An Atom Simulation
Make sure you have all the materials/a plan for working your SRP on Monday.
The Atom: Part 1
Here's the SIM for finishing the worksheet:
Build An Atom Simulation
Make sure you have all the materials/a plan for working your SRP on Monday.
Sections 3:
Here's the SIM for completing the worksheet:
Build An Atom Simulation
Make sure you have all the materials/a plan for working your SRP on Monday.
October Kairos:
Welcome back! Here's what you need to do by Tuesday:
-Study for the periodic table quiz you missed
-Be working on your Adopt an Element project (due Wednesday)
-Watch and take notes on this video:
-Check your email for your quia.com username and password (if you did not get it, send me an email). Then, locate the Quia class pages above for each section. Click on your section, and then click on the assignment posted there. You will need to sign in to complete it. You will have several attempts to answer the questions and you are welcome to use your textbook/notes.
Posted by
Ms. Tanzman
Thursday, October 3
Sections 3 & 6: Re-do the Quia homework AND watch and take notes on the following video:
The Atom: Part 1
The Atom: Part 1
Posted by
Ms. Tanzman
Wednesday, October 2
Sections 1, 2 & 7: Retake the Quia HW from last night
Section 3:
Section 3:
- Check your email for your Quia username and password
- Complete Quia HW
- Adopt an Element project due next Wednesday
- Test corrections due tomorrow
- Check your email for your Quia username and password
- Complete Quia HW
Posted by
Ms. Tanzman
Tuesday, October 1
Sections 1, 2 & 7:
1st Extra Credit Assignment (due Wednesday, 10/2): Read and outline the story on page 15 in your textbook and answer the two questions. This assignment must be typed and handed in at the beginning of class.
- Periodic table quiz tomorrow
- Complete your Quia homework (classpage can be found to the left)
- Adopt an Element project due next Wednesday
Section 6:
- Periodic table quiz tomorrow
- Adopt an Element project due next Wednesday
**Reminder:
Posted by
Ms. Tanzman
Monday, September 30
All: Quiz on the first 20 elements of the periodic table (symbol, atomic #, mass # and location) on Wednesday. See 'Dynamic Periodic Table' under Helpful Links for assistance.
Section 1: Finish your lab and complete your colored/annotated periodic table. Use the powerpoint at the bottom of the page and ptable.com.
Sections 2, 3 & 7: Complete your colored/annotated periodic table. Use the powerpoint at the bottom of the page and ptable.com.
Section 6: As per our discussion today in class, make sure all of the following is done by tomorrow, if you have not completed it already:
1) Complete your colored/annotated periodic table. Use the powerpoint at the bottom of the page and ptable.com.
2) Then read 16-20 and answer questions 1-5
3) Watch and take notes on the following video:
Intro to Chemistry
Section 1: Finish your lab and complete your colored/annotated periodic table. Use the powerpoint at the bottom of the page and ptable.com.
Sections 2, 3 & 7: Complete your colored/annotated periodic table. Use the powerpoint at the bottom of the page and ptable.com.
Section 6: As per our discussion today in class, make sure all of the following is done by tomorrow, if you have not completed it already:
1) Complete your colored/annotated periodic table. Use the powerpoint at the bottom of the page and ptable.com.
2) Then read 16-20 and answer questions 1-5
3) Watch and take notes on the following video:
Intro to Chemistry
Posted by
Ms. Tanzman
Thursday, September 26
Section 1: Test corrections are due on Monday!
Section 6: Read textbook pages 6-14 and answer questions 1-4 under section review. Then read 16-20 and answer questions 1-5
Section 6: Read textbook pages 6-14 and answer questions 1-4 under section review. Then read 16-20 and answer questions 1-5
Posted by
Ms. Tanzman
Wednesday, September 25
All: Read textbook pages 6-14 and answer questions 1-4 under section review. Then read 16-20 and answer questions 1-5
1st Extra Credit Assignment (due Wednesday, 10/2): Read and outline the story on page 15 in your textbook and answer the two questions. This assignment must be typed and handed in at the beginning of class.
All: Make sure your SRP binders are ready for tomorrow!
1st Extra Credit Assignment (due Wednesday, 10/2): Read and outline the story on page 15 in your textbook and answer the two questions. This assignment must be typed and handed in at the beginning of class.
All: Make sure your SRP binders are ready for tomorrow!
Posted by
Ms. Tanzman
Monday, September 23
Sections 1, 2 & 7: Watch and take notes on the following video:
Intro to Chemistry
**Make sure you're working on your project proposal!
Intro to Chemistry
**Make sure you're working on your project proposal!
Posted by
Ms. Tanzman
Friday, September 20
Sections 1, 2 & 7: Research project proposal due date has been pushed back. Enjoy your homework - free weekend!
Section 6: Study!! Look back over your notes and re-watch the videos. Make sure to check your email for the answer keys to the two worksheets from class today. Test topics are: measurement tools, conversions, scientific notation, sig figs, scientific method, density, accuracy, precision and percent error.
Here are some extra videos on what we learned today:
Accuracy vs. Precision
Percent error
Section 6: Study!! Look back over your notes and re-watch the videos. Make sure to check your email for the answer keys to the two worksheets from class today. Test topics are: measurement tools, conversions, scientific notation, sig figs, scientific method, density, accuracy, precision and percent error.
Here are some extra videos on what we learned today:
Accuracy vs. Precision
Percent error
Posted by
Ms. Tanzman
Thursday, September 19
Sections 1, 2 & 7: Study!! Look back over your notes and re-watch the videos. Make sure to check your email for the answer keys to the two worksheets from class today. Test topics are: measurement tools, conversions, scientific notation, sig figs, scientific method, density, accuracy, precision and percent error.
Here are some extra videos on what we learned today:
Percent error
Section 6: Complete the Sci Not/Sig Fig worksheet AND the textbook problems in your notebook.
Section 6: Complete the Sci Not/Sig Fig worksheet AND the textbook problems in your notebook.
Posted by
Ms. Tanzman
Wednesday, September 18
Section 1: Complete problems 34, 38-43, 45 - 48 and 58 on pages 60-61 of your textbook in your notebook. Remember that the answer to all calculation problems should have the correct number of sig figs!
1. A block of aluminum occupies a volume of 15.0 mL and weighs 40.5 g. What is its density?
2. Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder weighs 306.0 g. From this information, calculate the density of mercury.
3. What is the weight of the ethanol that exactly fills a 200.0 mL container?
The density of ethanol is 0.789 g/mL.
4. What volume of silver metal will weigh exactly 2500.0 g. The density of silver is 10.5 g/cm3.
Section 3: Study!! Look back over your notes and re-watch the videos. Make sure to check your email for the answer keys to the two worksheets from class today. I'll be in the SA office on 3L tomorrow morning if you need help.
Section 6: The two videos below will help you rewrite your post-lab answers with the correct number of sig figs.
Video 1 - this is a review of how to determine if a number is significant:
Determining Sig Figs
Video 2 - this video you MUST watch, as it contains the rules for sig figs when calculating a value:
Sig Fig Calculation Rules
Section 7: Complete the Accuracy & Precision AND the Chapter Review worksheets that you picked up today in class.
Posted by
Ms. Tanzman
Tuesday, September 17
Section 1: Complete your Sci Not/Sig Fig Worksheet (the videos below will help!) AND bring in an experiment from the internet that you'd like your group to do for the first section of your science fair project.
Determining Sig Figs
Sig Fig Calculation Rules
Determining Sig Figs
Sig Fig Calculation Rules
Section 2: Bring in an experiment from the internet that you'd like your group to do for the first section of your science fair project.
Section 3: Finish your Sci Not/Sig Fig worksheet. Also, complete problems 38-43, 45 - 48 on page 60 of your textbook in your notebook. Remember that the answer to all calculation problems should have the correct number of sig figs!
Also, watch the first 4 minutes of the video below - you do NOT need to take notes!
Density
Section 7: Finish your Sci Not/Sig Fig worksheet. Also, complete problems 38-43, 45 - 48 on page 60 of your textbook in your notebook. Remember that the answer to all calculation problems should have the correct number of sig figs!
Section 1, 2, 7: Test FRIDAY on measurement tools, scientific notation, sig figs, scientific method, density, accuracy and precision. Section 3: Test THURSDAY. Section 6: TEST MONDAY.
SCIENCE RESEARCH PROJECT TOPICS
Section 3: Finish your Sci Not/Sig Fig worksheet. Also, complete problems 38-43, 45 - 48 on page 60 of your textbook in your notebook. Remember that the answer to all calculation problems should have the correct number of sig figs!
Also, watch the first 4 minutes of the video below - you do NOT need to take notes!
Density
Section 7: Finish your Sci Not/Sig Fig worksheet. Also, complete problems 38-43, 45 - 48 on page 60 of your textbook in your notebook. Remember that the answer to all calculation problems should have the correct number of sig figs!
Section 1, 2, 7: Test FRIDAY on measurement tools, scientific notation, sig figs, scientific method, density, accuracy and precision. Section 3: Test THURSDAY. Section 6: TEST MONDAY.
SCIENCE RESEARCH PROJECT TOPICS
- Redox Fuel Cells(STEM)
- Organic Compounds
- Energy and Thermodynamics
- Chemical Analysis
- Food Analysis
- Solutions and Electrolytics
- Biofuel Extraction and Cell Creation
- Effects of Pesticides on the Environment and Health
- Solar Power
Posted by
Ms. Tanzman
Monday, September 16
Section 3: The two videos below will help you rewrite your post-lab answers with the correct number of sig figs.
Video 1 - this is a review of how to determine if a number is significant:
Determining Sig Figs
Video 2 - this video you MUST watch, as it contains the rules for sig figs when calculating a value:
Sig Fig Calculation Rules
Video 1 - this is a review of how to determine if a number is significant:
Determining Sig Figs
Video 2 - this video you MUST watch, as it contains the rules for sig figs when calculating a value:
Sig Fig Calculation Rules
Section 6: Finish your post-lab questions AND watch and take notes on the video below:
Posted by
Ms. Tanzman
Friday, September 13
Section 1: Complete your post-lab questions and don't forget to leave some room to the right of your answers for later amendment.
Section 2: The two videos below will help you rewrite your post-lab answers with the correct number of sig figs.
Video 1 - this is just a review of how to determine if a number is significant, if you feel you need more time with this concept:
Determining Sig Figs
Video 2 - this video you MUST watch, as it contains the rules for sig figs when calculating a value:
Sig Fig Calculation Rules
Side note - Dang autocorrect! too = two! Though they are almost 'too' great, right?... (Check your email if you want to get this joke)
Section 3: Finish your post-lab questions AND watch and take notes on the video below:
Scientific Notation and Sig Figs
Section 6: Complete your Metric Mania worksheet and the practice problems below (in your notebook):
1. 3.68 kg = __________ g
2. 568 cm = __________ m
3. 8700 ml = __________ l
4. 25 mg = __________ g
5. 0.101 cm = __________ mm
6. 250 ml = __________ l
7. 600 g = __________ kg
8. 8900 mm = __________ m
9. 0.000004 m = __________ mm
10. 0.250 kg = __________ mg
Section 2: The two videos below will help you rewrite your post-lab answers with the correct number of sig figs.
Video 1 - this is just a review of how to determine if a number is significant, if you feel you need more time with this concept:
Determining Sig Figs
Video 2 - this video you MUST watch, as it contains the rules for sig figs when calculating a value:
Sig Fig Calculation Rules
Side note - Dang autocorrect! too = two! Though they are almost 'too' great, right?... (Check your email if you want to get this joke)
Section 3: Finish your post-lab questions AND watch and take notes on the video below:
Scientific Notation and Sig Figs
Section 6: Complete your Metric Mania worksheet and the practice problems below (in your notebook):
1. 3.68 kg = __________ g
2. 568 cm = __________ m
3. 8700 ml = __________ l
4. 25 mg = __________ g
5. 0.101 cm = __________ mm
6. 250 ml = __________ l
7. 600 g = __________ kg
8. 8900 mm = __________ m
9. 0.000004 m = __________ mm
10. 0.250 kg = __________ mg
11. A block of
aluminum occupies a volume of 15.0 mL and weighs 40.5 g. What is its density?
12. Mercury metal is
poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used
to fill the cylinder weighs 306.0 g. From this information, calculate the
density of mercury.
13. What is the weight of the ethanol that
exactly fills a 200.0 mL container?
The density of
ethanol is 0.789 g/mL.
14. What volume of silver metal will weigh exactly 2500.0 g.
The density of silver is 10.5 g/cm3.
Section 7: The two videos below will help you rewrite your post-lab answers with the correct number of sig figs.
Video 1 - this is a review of how to determine if a number is significant:
Determining Sig Figs
Video 2 - this video you MUST watch, as it contains the rules for sig figs when calculating a value:
Sig Fig Calculation Rules
Section 7: The two videos below will help you rewrite your post-lab answers with the correct number of sig figs.
Video 1 - this is a review of how to determine if a number is significant:
Determining Sig Figs
Video 2 - this video you MUST watch, as it contains the rules for sig figs when calculating a value:
Sig Fig Calculation Rules
Posted by
Ms. Tanzman
Thursday, September 12
Sections 1, 2 & 7: Watch and take notes on the following video:
Scientific Notation and Sig Figs
Section 6: Watch the following video and take notes/fill out your worksheet with the corresponding information
.https://www.youtube.com/watch?v=WuaxXcgX6Rc
Scientific Notation and Sig Figs
Section 6: Watch the following video and take notes/fill out your worksheet with the corresponding information
.https://www.youtube.com/watch?v=WuaxXcgX6Rc
Posted by
Ms. Tanzman
Wednesday, September 11
Section 1: Complete your "Metric Mania" worksheet and the following problems in your notebook:
1. 3.68 kg = __________ g
2. 568 cm = __________ m
3. 8700 ml = __________ l
4. 25 mg = __________ g
5. 0.101 cm = __________ mm
6. 250 ml = __________ l
7. 600 g = __________ kg
8. 8900 mm = __________ m
9. 0.000004 m = __________ mm
10. 0.250 kg = __________ mg
Section 2: Complete the "Metric Mania" worksheet you received in class today.
Section 3: Watch the following video and take notes/fill out your worksheet with the corresponding information.
https://www.youtube.com/watch?v=WuaxXcgX6Rc
Section 6: The Mythbusters video link is below, watch it and fill out the rest of the corresponding worksheet.
http://dsc.discovery.com/tv-shows/mythbusters/videos/throw-like-a-girl-results.htm
Also, create a measurement system to re-"measure" the item you measured last night but it cannot be based on ANY formal measurement system. Bring your measurement tool and item (if you can) into class tomorrow.
Section 7: Complete the back of your "Metric Mania" worksheet AND your post lab questions 3-10. Don't forget to leave a little bit of space to the right of your post lab questions!
1. 3.68 kg = __________ g
2. 568 cm = __________ m
3. 8700 ml = __________ l
4. 25 mg = __________ g
5. 0.101 cm = __________ mm
6. 250 ml = __________ l
7. 600 g = __________ kg
8. 8900 mm = __________ m
9. 0.000004 m = __________ mm
10. 0.250 kg = __________ mg
Section 2: Complete the "Metric Mania" worksheet you received in class today.
Section 3: Watch the following video and take notes/fill out your worksheet with the corresponding information.
https://www.youtube.com/watch?v=WuaxXcgX6Rc
Section 6: The Mythbusters video link is below, watch it and fill out the rest of the corresponding worksheet.
http://dsc.discovery.com/tv-shows/mythbusters/videos/throw-like-a-girl-results.htm
Also, create a measurement system to re-"measure" the item you measured last night but it cannot be based on ANY formal measurement system. Bring your measurement tool and item (if you can) into class tomorrow.
Section 7: Complete the back of your "Metric Mania" worksheet AND your post lab questions 3-10. Don't forget to leave a little bit of space to the right of your post lab questions!
Posted by
Ms. Tanzman
Tuesday, September 10
Sections 1, 2 & 7: Watch and take notes on the following video:
Sections 3: Complete your Mythbusters worksheet.
Also, create a measurement system to re-"measure" the item you measured last night but it cannot be based on ANY formal measurement system. Bring your measurement tool and item into class tomorrow.
Section 6: Your first assignment is to find an object in your house that you can measure in three different ways. What the heck does that mean??? Well, think of yourself, for example. You can tell someone how tall you are, how heavy you are, how much space you take up. Even though all of these things are descriptive of you, they would all be measured in very different ways.
Once you've chosen your object, write in your binder the following information:Section 6: Your first assignment is to find an object in your house that you can measure in three different ways. What the heck does that mean??? Well, think of yourself, for example. You can tell someone how tall you are, how heavy you are, how much space you take up. Even though all of these things are descriptive of you, they would all be measured in very different ways.
What is it?
Why did you choose it?
1st measurement and what instrument/method you used to obtain it.
2nd measurement and what instrument/method you used to obtain it.
3rd measurement and what instrument/method you used to obtain it.
**Make sure to include units and be as accurate as possible!
Posted by
Ms. Tanzman
Monday, September 9
Sections 1&2: Take two of the items you measured last night and "measure" them again, but this time you are not allowed to use any formal measurement system - you must create your own!! If you cannot bring in your created measurement tool, make sure to take a picture on your phone while you're measuring! Think outside the box!!
Section 3 & 7: Please bring in the item you measured last night, or another item that can be easily measured. Bringing in your measured item from last night's homework is ideal!
Also, watch this video:
http://dsc.discovery.com/tv-shows/mythbusters/videos/throw-like-a-girl-results.htm
Section 3 & 7: Please bring in the item you measured last night, or another item that can be easily measured. Bringing in your measured item from last night's homework is ideal!
Also, watch this video:
http://dsc.discovery.com/tv-shows/mythbusters/videos/throw-like-a-girl-results.htm
Posted by
Ms. Tanzman
Welcome to Chemistry!
This webpage is going to be your key to success in chemistry. Everything you need, you can find here! You should check the website EVERYDAY! (In fact, bookmark it right now).
Your first assignment is to find an object in your house that you can measure in three different ways. What the heck does that mean??? Well, think of yourself, for example. You can tell someone how tall you are, how heavy you are, how much space you take up. Even though all of these things are descriptive of you, they would all be measured in very different ways.
Once you've chosen your object, write in your binder the following information:
What is it?
Why did you choose it?
1st measurement and what instrument/method you used to obtain it.
2nd measurement and what instrument/method you used to obtain it.
3rd measurement and what instrument/method you used to obtain it.
**Make sure to include units and be as accurate as possible!
Your first assignment is to find an object in your house that you can measure in three different ways. What the heck does that mean??? Well, think of yourself, for example. You can tell someone how tall you are, how heavy you are, how much space you take up. Even though all of these things are descriptive of you, they would all be measured in very different ways.
Once you've chosen your object, write in your binder the following information:
What is it?
Why did you choose it?
1st measurement and what instrument/method you used to obtain it.
2nd measurement and what instrument/method you used to obtain it.
3rd measurement and what instrument/method you used to obtain it.
**Make sure to include units and be as accurate as possible!
Posted by
Ms. Tanzman
Final
Answer key is up (homework docs) and here's the link to the ppts:
http://www.edline.net/pages/xhs/Classes/1213_207/Powerpoints
See you all in the morning! Come well rested!
http://www.edline.net/pages/xhs/Classes/1213_207/Powerpoints
See you all in the morning! Come well rested!
Posted by
Ms. Tanzman
Review Session!
All: Tomorrow's review session will be from 11:30-12:30 in room 3K2.
Posted by
Ms. Tanzman
Tuesday, June 4
Sections 9 & 11: Complete your review packet.
Section 10: Great come-back win today by the Flying Purple Uni-Dragons! And, a consistently well-played game by the Rubber Duckies from Hell! For homework tonight, complete the final review sheet (can be found under homework docs) - tomorrow is our only day to review, come with questions!
All: If you have a moment, please fill out this teacher evaluation. It is anonymous and I appreciate you being as honest as possible. Thanks!
http://www.quia.com/sv/582146.html
Section 10: Great come-back win today by the Flying Purple Uni-Dragons! And, a consistently well-played game by the Rubber Duckies from Hell! For homework tonight, complete the final review sheet (can be found under homework docs) - tomorrow is our only day to review, come with questions!
All: If you have a moment, please fill out this teacher evaluation. It is anonymous and I appreciate you being as honest as possible. Thanks!
http://www.quia.com/sv/582146.html
Posted by
Ms. Tanzman
Monday, June 3
Section 8: Congratulations to Team Yoda on a strong win today! For homework tonight, complete the final review sheet (can be found under homework docs) - tomorrow is our only day to review, come with questions!
Section 9: Congratulations to The Sith Lords (and Ben Futterman) for a great come-back win today, and to The Juans for a consistently excellent game!
For homework tonight, answer questions 1-8 on the final review sheet (can be found under homework docs).
Section 11: Great start to our showdown today! Operation Milk and Cookies will have to come in with a strong start tomorrow, if they hope to catch Dirty Mike and the Boyz. We will start class with Jake and Jarred in the hot seats.
For homework tonight, print out and answer questions 1-8 on the final review sheet (can be found under homework docs). Make sure to bring the review sheet to class tomorrow.
**All: Due to unforeseen circumstances, tomorrow's review session has been moved to the MORNING (7:40) - still in 3K2.
Section 9: Congratulations to The Sith Lords (and Ben Futterman) for a great come-back win today, and to The Juans for a consistently excellent game!
For homework tonight, answer questions 1-8 on the final review sheet (can be found under homework docs).
Section 11: Great start to our showdown today! Operation Milk and Cookies will have to come in with a strong start tomorrow, if they hope to catch Dirty Mike and the Boyz. We will start class with Jake and Jarred in the hot seats.
For homework tonight, print out and answer questions 1-8 on the final review sheet (can be found under homework docs). Make sure to bring the review sheet to class tomorrow.
**All: Due to unforeseen circumstances, tomorrow's review session has been moved to the MORNING (7:40) - still in 3K2.
Posted by
Ms. Tanzman
Friday, May 31
Sections 8 & 10: Under 'Homework Documents' you will find a sheet with 20 different hydrocarbon structures. The sheet you picked up at the end of the day has 20 spots to fill in the names of these structures. You should complete numbers 1-4, 7-16, 18 & 20. DON'T FORGET your cis and trans prefixes where they apply!
Sections 9 & 11: Complete your hydrocarbon naming worksheet. Also, under 'Homework Documents' you will find a sheet with 20 different hydrocarbon structures. You should complete numbers 1-4, 11, 12, 16, 18 & 20 in your notebook. DON'T FORGET your cis and trans prefixes where they apply!
All: The final review sheet sheet can be found under homework docs
Sections 9 & 11: Complete your hydrocarbon naming worksheet. Also, under 'Homework Documents' you will find a sheet with 20 different hydrocarbon structures. You should complete numbers 1-4, 11, 12, 16, 18 & 20 in your notebook. DON'T FORGET your cis and trans prefixes where they apply!
All: The final review sheet sheet can be found under homework docs
Posted by
Ms. Tanzman
Thursday, May 30
Section 8: Make sure both worksheets from class are completed. All cyclo-hydrocarbons can be done for extra credit.
Section 10: Complete your naming hyrdocarbons worksheet. All cyclo-hydrocarbons can be done for extra credit.
Sections 9 & 11: Complete the worksheet you got today in class. You DO NOT have to answer the first two problems on the bottom of the first page or #8 on the back.
Section 10: Complete your naming hyrdocarbons worksheet. All cyclo-hydrocarbons can be done for extra credit.
Sections 9 & 11: Complete the worksheet you got today in class. You DO NOT have to answer the first two problems on the bottom of the first page or #8 on the back.
Posted by
Ms. Tanzman
Wednesday, May 29
Section 8: Complete the homework listed for sections 9 & 11 yesterday.
Section 10:
Section 10:
1) Read pages 711-714 and answer questions 1-5 on page 714.
2) Try problems 6 & 7 from the back of today's worksheet
3) The quia assignment has been re-opened. You have until class-time tomorrow to retake it, if necessary.
Posted by
Ms. Tanzman
Tuesday, May 28
Section 9 & 11: Read pages 711-714 and answer questions 1-5 on page 714.
Also, name/draw these:
1) 3-pentyne
2) 2-hexene
3) decane
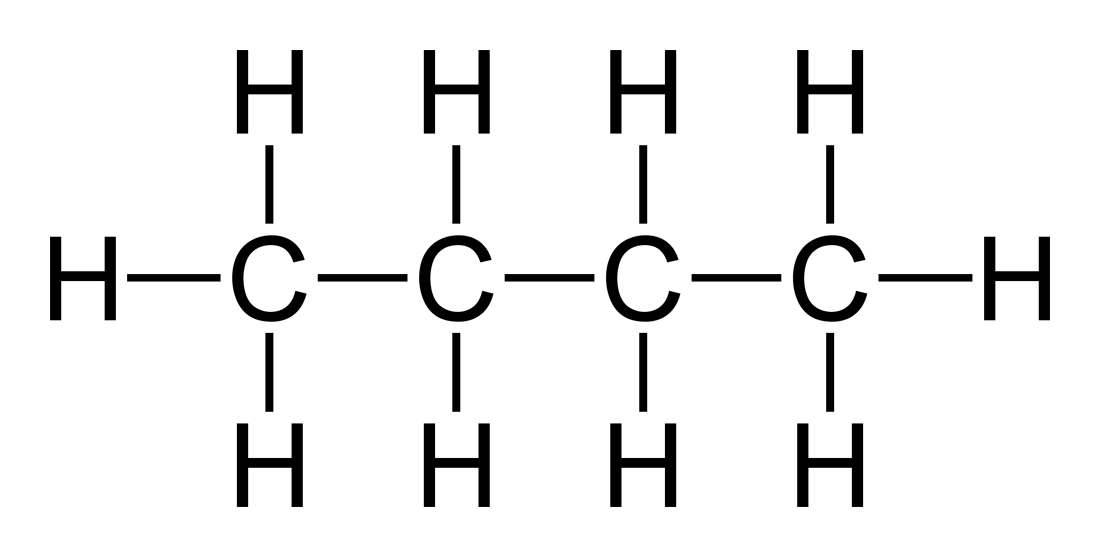
4)
5)
6)
Section 10: Here's the link to the quia assignment. Don't forget you have 2 attempts. YOU MUST SUBMIT YOUR ASSIGNMENT BY 8AM TOMORROW MORNING.
http://www.quia.com/pages/jtanzman11/page2
Also, name/draw these:
1) 3-pentyne
2) 2-hexene
3) decane
4)

5)
6)
Section 10: Here's the link to the quia assignment. Don't forget you have 2 attempts. YOU MUST SUBMIT YOUR ASSIGNMENT BY 8AM TOMORROW MORNING.
http://www.quia.com/pages/jtanzman11/page2
Posted by
Ms. Tanzman
Friday, May 24
Sections 8 & 9: Complete all of the following:
1) The first page of the half-life worksheet (I will collect it). The second page is practice for you, the answer key can be found at the bottom of the page.
2) Complete the fission/fusion assignment (Will also be collected). Here's the link that's at the top of the page:
http://science.howstuffworks.com/fusion-reactor.htm
This one will help too.... http://science.howstuffworks.com/nuclear-power.htm
3) Once you've done these two assignments, complete the quia homework. Don't forget, you have two tries to get a high score. YOU MUST COMPLETE THIS ASSIGNMENT BY MONDAY AT MIDNIGHT.
Section 8 link - http://www.quia.com/pages/jtanzman11/chem08
Section 9 link -http://www.quia.com/pages/jtanzman11/page1
Section 10: Complete your nuclear decay and fission/fusion worksheets. Here's the link that's at the top of the page: http://science.howstuffworks.com/fusion-reactor.htm
This one will help too.... http://science.howstuffworks.com/nuclear-power.htm
Section 11: Complete the fission/fusion assignment (Will also be collected). Here's the link that's at the top of the page: http://science.howstuffworks.com/fusion-reactor.htm
This one will help too.... http://science.howstuffworks.com/nuclear-power.htm
Also, below is the link to your quia homework. Don't forget, you have two tries to get a high score. YOU MUST COMPLETE THIS ASSIGNMENT BY MONDAY AT MIDNIGHT.
http://www.quia.com/pages/jtanzman11/page3
All: This video is great!: https://www.youtube.com/watch?v=mBdVK4cqiFs
1) The first page of the half-life worksheet (I will collect it). The second page is practice for you, the answer key can be found at the bottom of the page.
2) Complete the fission/fusion assignment (Will also be collected). Here's the link that's at the top of the page:
http://science.howstuffworks.com/fusion-reactor.htm
This one will help too.... http://science.howstuffworks.com/nuclear-power.htm
3) Once you've done these two assignments, complete the quia homework. Don't forget, you have two tries to get a high score. YOU MUST COMPLETE THIS ASSIGNMENT BY MONDAY AT MIDNIGHT.
Section 8 link - http://www.quia.com/pages/jtanzman11/chem08
Section 9 link -http://www.quia.com/pages/jtanzman11/page1
Section 10: Complete your nuclear decay and fission/fusion worksheets. Here's the link that's at the top of the page: http://science.howstuffworks.com/fusion-reactor.htm
This one will help too.... http://science.howstuffworks.com/nuclear-power.htm
Section 11: Complete the fission/fusion assignment (Will also be collected). Here's the link that's at the top of the page: http://science.howstuffworks.com/fusion-reactor.htm
This one will help too.... http://science.howstuffworks.com/nuclear-power.htm
Also, below is the link to your quia homework. Don't forget, you have two tries to get a high score. YOU MUST COMPLETE THIS ASSIGNMENT BY MONDAY AT MIDNIGHT.
http://www.quia.com/pages/jtanzman11/page3
All: This video is great!: https://www.youtube.com/watch?v=mBdVK4cqiFs
Posted by
Ms. Tanzman
Thursday, May 23
Section 9: Complete your nuclear decay worksheet
Sections 8 & 11: Complete your graph from today's activity AND the nuclear decay worksheet.
All: Here are the answers from 3b&c of last night's homework, and 1&2 from tonight's, to help you get started:
Sections 8 & 11: Complete your graph from today's activity AND the nuclear decay worksheet.
All: Here are the answers from 3b&c of last night's homework, and 1&2 from tonight's, to help you get started:
Posted by
Ms. Tanzman
Wednesday, May 22
All Sections: Watch and take notes on these videos:
Alpha Decay
Beta Decay
Then, answer questions 1-3(all parts) on page 684 of your textbook. You may need to do some reading of that section to answer the questions.
Alpha Decay
Beta Decay
Then, answer questions 1-3(all parts) on page 684 of your textbook. You may need to do some reading of that section to answer the questions.
Posted by
Ms. Tanzman
Tuesday, May 21
All Sections: At the bottom of the page you will find the Nuclear Chemistry powerpoint. Please take notes, in your notebook, on the first 11 slides. We will discuss them tomorrow, so you need to have them already available to you. Printing out the slides will only hinder your ability to remember the material.
Extra credit 'quiz a' can be taken on Thursday, before or after school in room 3K2.
Extra credit 'quiz a' can be taken on Thursday, before or after school in room 3K2.
Posted by
Ms. Tanzman
Monday, May 20
Sections 8 & 10: Study for your redox quiz! Answer key to today's worksheet has been emailed to you. Powerpoint is at the bottom of the page.
Posted by
Ms. Tanzman
Friday, May 17
Section 9: Complete the worksheet you picked up at the end of class.
Sections 10 & 11: Finish your redox worksheet and write half reactions (on the sheet or in your notebook) for 9b, c, f,g, 10c & d. **Check yesterday's blog post for the answer to 9b**
Images from today's class (many thanks to Donovan S for taking the pictures!):
Sections 10 & 11: Finish your redox worksheet and write half reactions (on the sheet or in your notebook) for 9b, c, f,g, 10c & d. **Check yesterday's blog post for the answer to 9b**
Images from today's class (many thanks to Donovan S for taking the pictures!):
Posted by
Ms. Tanzman
Thursday, May 16
Sections 8 & 9: Finish your redox worksheet. Here's the answer to 9b to help you along:
Section 10: Outline/take notes on pgs 637 - 641 in your textbook AND answer section review questions 1-3 on page 641.
Section 10: Outline/take notes on pgs 637 - 641 in your textbook AND answer section review questions 1-3 on page 641.
Posted by
Ms. Tanzman
Wednesday, May 15
Sections 8, 9 & 11: Outline/take notes on pgs 637 - 641 in your textbook AND answer section review questions 1-3 on page 641.
Seniors taking the final: Your exam is on Monday, May 20th at 12:35 in room 4L5. I will be available for review after school on Friday in room 3K2.
Seniors taking the final: Your exam is on Monday, May 20th at 12:35 in room 4L5. I will be available for review after school on Friday in room 3K2.
Posted by
Ms. Tanzman
Tuesday, May 14
All Sections: Use pages 631-635 of your textbook to answer questions 1-7 from the redox reactions worksheet.
(extra copies of the worksheet can be found under homework documents)
Section 9: Check your email.
(extra copies of the worksheet can be found under homework documents)
Section 9: Check your email.
Posted by
Ms. Tanzman
Friday, May 10
Section 8: Complete problems 1-7 on your Specific Heat/Enthalpy "quiz" (the worksheet I handed out at the end of class). You can find the specific heat capacity values you will need on page 533 of your textbook.
Posted by
Ms. Tanzman
Thursday, May 9
Sections 9 & 10: Complete problems 1-7 on your Specific Heat/Enthalpy "quiz" (the worksheet I handed out at the end of class)
Posted by
Ms. Tanzman
Wednesday, May 8
Sections 8-10: Complete the Hess's Law Worksheet (2)
Section 11: Complete the Hess's Law Worksheet (2) and #1-3 & 7 on the Specific Heat/Enthalpy "quiz"
Section 11: Complete the Hess's Law Worksheet (2) and #1-3 & 7 on the Specific Heat/Enthalpy "quiz"
Posted by
Ms. Tanzman
Tuesday, May 7
Section 8: Complete #1 and 2 ONLY on your Hess's Law worksheet. Do your work on scrap paper (or the back) so that it is legible!
Sections 9 & 11: Complete ALL of the Hess's Law worksheet we started today in class. Do your work on scrap paper (or the back) so that it is legible!
Sections 9 & 11: Complete ALL of the Hess's Law worksheet we started today in class. Do your work on scrap paper (or the back) so that it is legible!
Posted by
Ms. Tanzman
Monday, May 6
All Juniors: Please watch or finish watching the video on conflict minerals:
We will debate next week, (and I will expect you to be able to cite points from the second half of the film) so here is a reminder of what was on last Monday's post:
Based on your reading (found to the left) and today's video, answer the following questions:
2) Which of the aforementioned parties do you think have the loudest voice?
3) Is there a way for the scientific community to remain patriotic and pacifistic?
Be prepared to debate your answers next week.
Posted by
Ms. Tanzman
Friday, May 3
All Sections: Quiz corrections due Monday
Juniors: Be ready for Monday's debate!
Section 10: Answer question #1 on the worksheet you got today in class.
Juniors: Be ready for Monday's debate!
Section 10: Answer question #1 on the worksheet you got today in class.
Posted by
Ms. Tanzman
Thursday, May 2
Section 8: Follow the instructions posted for sections 9-11 yesterday.
Posted by
Ms. Tanzman
Wednesday, May 1
Sections 9-11: Complete the front (if you have not already) and back of the first page of your pink packet. Here's the answer the the first problem on the back page, to help get you started:
Posted by
Ms. Tanzman
Tuesday, April 30
All Sections: Read textbook page 531-544. Then, answer questions 1-5 on page 544.
Posted by
Ms. Tanzman
Monday, April 29
Sections 8, 9 & 11: Based on your reading (found to the left) and today's video, answer the following questions:
2) Which of the aforementioned parties do you think have the loudest voice?
3) Is there a way for the scientific community to remain patriotic and pacifistic?
Be prepared to debate your answers next week.
Posted by
Ms. Tanzman
Thursday, April 25
Section 8: Today, we started out 'lab' by added 15mL of 1.0 M HCl to a flask. Then we added 75 mL of water.
After this dilution, what is the new molarity of the HCl solution?
If we used 150 mL of NaOH to reach the endpoint, what was the molarity of the NaOH solution?
Now that you have those numbers, I want you to think about the question I asked you today in class, did the addition of the water effect the outcome of the titration?
Section 9: Complete the worksheet I handed you in class today. Be prompt for class tomorrow.
Sections 8, 10 and 11: Tomorrow you should come straight to the commons during our class period. I will be near the 1L entrance. Make sure you come straight to find me so I can take attendance and hand you the worksheet you will be doing while at the science fair. DO NOT BE LATE.
**If you are taking a make-up quiz tomorrow meet me in 3K2.
After this dilution, what is the new molarity of the HCl solution?
If we used 150 mL of NaOH to reach the endpoint, what was the molarity of the NaOH solution?
Now that you have those numbers, I want you to think about the question I asked you today in class, did the addition of the water effect the outcome of the titration?
Section 9: Complete the worksheet I handed you in class today. Be prompt for class tomorrow.
Sections 8, 10 and 11: Tomorrow you should come straight to the commons during our class period. I will be near the 1L entrance. Make sure you come straight to find me so I can take attendance and hand you the worksheet you will be doing while at the science fair. DO NOT BE LATE.
**If you are taking a make-up quiz tomorrow meet me in 3K2.
Posted by
Ms. Tanzman
Wednesday, April 24
Sections 8, 10 & 11: Study for your quiz tomorrow! Don't forget to review your acid and base naming! Here's a link to help:
https://www.boundless.com/chemistry/atoms-molecules-and-ions/naming-compounds/naming-acids-and-bases/
The answer keys to your practice pages can be found under homework docs. **pH key 3 is for section 10 only!**
I will be in 3K2 at 7:45 tomorrow if you have questions.
You will NOT be getting equations on the quiz!
https://www.boundless.com/chemistry/atoms-molecules-and-ions/naming-compounds/naming-acids-and-bases/
The answer keys to your practice pages can be found under homework docs. **pH key 3 is for section 10 only!**
I will be in 3K2 at 7:45 tomorrow if you have questions.
You will NOT be getting equations on the quiz!
Posted by
Ms. Tanzman
Tuesday, April 23
Sections 8, 10 & 11: Quiz Thursday on calculating concentrations and pH.
Sections 9-11: Complete the worksheet you received in class today. This video will help:
https://www.youtube.com/watch?v=o6WCY1ihUvQ
Sections 9-11: Complete the worksheet you received in class today. This video will help:
https://www.youtube.com/watch?v=o6WCY1ihUvQ
Posted by
Ms. Tanzman
Monday, April 22
Happy Earth Day!
http://www.50waystohelp.com/
Section 8: We started today's video at 16:00 (can be found below). Reading will be up tomorrow, you should use it to add to your note taking sheet.
The Disappearing Spoon
Section 9: Answer the following questions:
1) How are the various ways we think water formed on early Earth?
2) Describe the theory of water delivery by comet to early Earth.
3) What are the flaws in this theory? Explain and give proof.
4) Look up "heavy water" and give a brief description of it.
5) What are some current and historical uses of heavy water?
http://www.pbs.org/wgbh/nova/space/origins-series-overview.html#origins-earth-born
Section 10: Answer the following questions:
1) How did Newton picture space?
2) Describe Newtonian space and its interactions with the objects in it.
3) How would Newton explain the skaters arms splaying out as she spins?
4) What is special about the speed of light?
http://www.pbs.org/wgbh/nova/physics/fabric-of-cosmos.html#fabric-space
Section 11: Don't forget to send me your data!
Also, look up the difference between distilled and deionized water and write a short explanation in your notebook. Then, explain how/if you think the experiment would have changed if we used distilled, deionized or tap water.
Finally, think about this:
What was the composition of your solution when it reached its endpoint? What types of ions were in there? Was it acidic? Basic? Neutral?
http://www.50waystohelp.com/
Section 8: We started today's video at 16:00 (can be found below). Reading will be up tomorrow, you should use it to add to your note taking sheet.
The Disappearing Spoon
Section 9: Answer the following questions:
1) How are the various ways we think water formed on early Earth?
2) Describe the theory of water delivery by comet to early Earth.
3) What are the flaws in this theory? Explain and give proof.
4) Look up "heavy water" and give a brief description of it.
5) What are some current and historical uses of heavy water?
http://www.pbs.org/wgbh/nova/space/origins-series-overview.html#origins-earth-born
Section 10: Answer the following questions:
1) How did Newton picture space?
2) Describe Newtonian space and its interactions with the objects in it.
3) How would Newton explain the skaters arms splaying out as she spins?
4) What is special about the speed of light?
http://www.pbs.org/wgbh/nova/physics/fabric-of-cosmos.html#fabric-space
Section 11: Don't forget to send me your data!
Also, look up the difference between distilled and deionized water and write a short explanation in your notebook. Then, explain how/if you think the experiment would have changed if we used distilled, deionized or tap water.
Finally, think about this:
What was the composition of your solution when it reached its endpoint? What types of ions were in there? Was it acidic? Basic? Neutral?
Posted by
Ms. Tanzman
Friday, April 19
Sections 8 & 11: Complete your pH calculations worksheet you started in class today.
All: Don't forget that project boards are due on Tuesday!
All: Don't forget that project boards are due on Tuesday!
Posted by
Ms. Tanzman
Thursday, April 18
All: When completing your SFP paper keep the following in mind:
- You need to include EVERY part of the project. If you lost the packet I gave out at the beginning of the year, you can find it below under Homework Documents.
- Bullet points are not acceptable, except if you want to do a step-wise procedure section.
- Data tables and graphs should have TITLES, AXIS LABELS AND DESCRIPTIONS.
- I cannot seem to stress this enough, DATA TABLES SHOULD BE INTEGRATED INTO YOUR DISCUSSION.
- You must have an abstract (very short - 200 words)
- Include pictures! (label them as 'Figure 1.', 'Figure 2.', etc.
- IF THERE ARE NOT APPROPRIATE CITATIONS IN YOUR PAPER, YOU WILL RECEIVE A ZERO. THIS IS NOT UP FOR DISCUSSION.
- Your conclusion can include how you would modify the experiment to improve it in the future.
Posted by
Ms. Tanzman
Wednesday, April 17
All Sections: Complete the neutralization reactions worksheet (you either received it in class or can find it at the bottom of the webpage under homework docs).
**Final drafts should include ALL parts of your project. Due date has been moved to Friday at 4pm (hard copy or email).
**Final drafts should include ALL parts of your project. Due date has been moved to Friday at 4pm (hard copy or email).
Posted by
Ms. Tanzman
Tuesday, April 16
Sections 8 & 10: Use chapter 14, section 1 of your textbook to answer section review questions 1-5 on page 476.
Posted by
Ms. Tanzman
Friday, April 12
Sections 9 & 11: Use chapter 14, section 1 of your textbook to answer section review questions 1-5 on page 476.
Science Fair Project Update:
All final drafts are due on Thursday, April 18 - no extensions will be awarded. If you want me to review your draft, edit or help you in any way, I am free Monday from 2:45 -4 in the LC and Tuesday from 2:35-3:30 in 3K2. You can also make an appointment to meet with me during the day.
Early next week, I will be asking some of you to present at the science fair, please be sure to be checking your email.
Science Fair Project Update:
All final drafts are due on Thursday, April 18 - no extensions will be awarded. If you want me to review your draft, edit or help you in any way, I am free Monday from 2:45 -4 in the LC and Tuesday from 2:35-3:30 in 3K2. You can also make an appointment to meet with me during the day.
Early next week, I will be asking some of you to present at the science fair, please be sure to be checking your email.
Posted by
Ms. Tanzman
Thursday, April 11
Section 8: Complete the concept map that you picked up at the end of class.
Also, here's a game to help you study:
Solutions - Rags to Riches
Tomorrow's quiz includes:
-solution term definitions and concept questions
-solubility curve
-calculating molarity and dilutions
-colligative properties
The powerpoint is at the bottom of the website....good luck!
Also, here's a game to help you study:
Solutions - Rags to Riches
Tomorrow's quiz includes:
-solution term definitions and concept questions
-solubility curve
-calculating molarity and dilutions
-colligative properties
The powerpoint is at the bottom of the website....good luck!
Posted by
Ms. Tanzman
Wednesday, April 10
Section 8:
*Quiz next class
Section 10: Complete your concept map. *Quiz next class.
- Complete the dilutions side of the worksheet we started today in class.
- Next, click the link below, it will take you to a video on colligative properties. Take notes on the first 3 minutes and 48 seconds (up until osmotic pressure). You need to watch the section on osmotic pressure (until 8:58) but you do not need to take notes on it.
- Then, using all your notes, complete the back of your dilutions worksheet (heading says "solutions quiz")
- *Quiz on Friday
*Quiz next class
Section 10: Complete your concept map. *Quiz next class.
Posted by
Ms. Tanzman
Tuesday, April 9
Sections 8: Finish your phet simulation lab (including the post lab questions).
Sections 9 & 11: First, follow this link, it will bring you to a set of digital flash cards. Use the cards to fill out the solutions definition worksheet.
Then, watch and take notes on this video:
Molarity vs. Molality
(if you're having trouble accessing the video, you can go to khanacademy.org and search for 'molarity vs. molality')
Next, click the link below, it will take you to a video on colligative properties. Take notes on the first 3 minutes and 48 seconds (up until osmotic pressure). You need to watch the section on osmotic pressure (until 8:58) but you do not need to take notes on it.
Colligative Properties
Finally, see how much you know about solutions by playing this game:
Solutions - Rags to Riches
Section 10: Finish the worksheet you started today in class.
All: Quiz on Thursday
Sections 9 & 11: First, follow this link, it will bring you to a set of digital flash cards. Use the cards to fill out the solutions definition worksheet.
Then, watch and take notes on this video:
Molarity vs. Molality
(if you're having trouble accessing the video, you can go to khanacademy.org and search for 'molarity vs. molality')
Next, click the link below, it will take you to a video on colligative properties. Take notes on the first 3 minutes and 48 seconds (up until osmotic pressure). You need to watch the section on osmotic pressure (until 8:58) but you do not need to take notes on it.
Colligative Properties
Finally, see how much you know about solutions by playing this game:
Solutions - Rags to Riches
Section 10: Finish the worksheet you started today in class.
All: Quiz on Thursday
Posted by
Ms. Tanzman
Monday, April 8
Section 10:
Then, watch and take notes on this video:
Molarity vs. Molality
(if you're having trouble accessing the video, you can go to khanacademy.org and search for 'molarity vs. molality')
Next, click the link below, it will take you to a video on colligative properties. Take notes on the first 3 minutes and 48 seconds (up until osmotic pressure). You need to watch the section on osmotic pressure (until 8:58) but you do not need to take notes on it.
Colligative Properties
Finally, see how much you know about solutions by playing this game:
Solutions - Rags to Riches
First, follow this link, it will bring you to a set of digital flash cards. Use the cards to fill out the solutions definition worksheet.
Then, watch and take notes on this video:
Molarity vs. Molality
(if you're having trouble accessing the video, you can go to khanacademy.org and search for 'molarity vs. molality')
Next, click the link below, it will take you to a video on colligative properties. Take notes on the first 3 minutes and 48 seconds (up until osmotic pressure). You need to watch the section on osmotic pressure (until 8:58) but you do not need to take notes on it.
Colligative Properties
Finally, see how much you know about solutions by playing this game:
Solutions - Rags to Riches
Posted by
Ms. Tanzman
Wednesday, March 27
Sections 9-11: Complete the post-lab page from your phet simulation packet.
Have a great break!
Have a great break!
Posted by
Ms. Tanzman
Tuesday, March 26
Section 8: Complete the solubility curve worksheet that you picked up at the end of class today.
Section 9: Use chapter 12, section 2 of your textbook to answer questions 1-7 on page 416.
Sections 10 & 11: Complete the phet simulation packet from class today, including post-lab questions 1-5.
All: **Reminder** Data tables will now be due with your Discussion section on 4/9. Remember that data tables should be logically integrated into your discussion section.
Section 9: Use chapter 12, section 2 of your textbook to answer questions 1-7 on page 416.
Sections 10 & 11: Complete the phet simulation packet from class today, including post-lab questions 1-5.
Posted by
Ms. Tanzman
Monday, March 25
Sections 8 & 11: Write a one page write up on today's video. (due tomorrow!)
Section 9 (Juniors only): Complete the phet simulation packet from class today, including post-lab questions 1-6 (due tomorrow).
All: Data tables will now be due with your Discussion section on 4/9. Remember that data tables should be logically integrated into your discussion section.
Also, here how to find the new curved scores for your Gas Laws Test:
Section 9 (Juniors only): Complete the phet simulation packet from class today, including post-lab questions 1-6 (due tomorrow).
All: Data tables will now be due with your Discussion section on 4/9. Remember that data tables should be logically integrated into your discussion section.
Also, here how to find the new curved scores for your Gas Laws Test:
RAW score
|
CURVED score
|
RAW score
|
CURVED score
|
22
|
29
|
37
|
40.25
|
23
|
29.75
|
38
|
41
|
24
|
30.5
|
39
|
41.75
|
25
|
31.25
|
40
|
42.5
|
26
|
32
|
41
|
43.25
|
27
|
32.75
|
42
|
44
|
28
|
33.5
|
43
|
44.75
|
29
|
34.25
|
44
|
45.5
|
30
|
35
|
45
|
46.25
|
31
|
35.75
|
46
|
47
|
32
|
36.5
|
47
|
47.75
|
33
|
37.25
|
48
|
48.5
|
34
|
38
|
49
|
49.25
|
35
|
38.75
|
50
|
50
|
36
|
40
|
Posted by
Ms. Tanzman
Subscribe to:
Comments (Atom)















